| Books |
 |
 |
 |
 |
| 2004 |
2008 |
2013 |
2014 |
| |
 |
 |
 |
|
| 2015 |
2016 |
2019 |
|
| |
| |
| Pierre H. Dixneuf Research Topics |
Our objectives aim at new developments of organometallic catalysis, at the selective activation of inert bonds and simple molecules to create new green synthetic methods, with environment friendly reagents, under mild conditions and with atom economy and for the synthesis of target useful molecules, functional ligands or molecular materials of industrial interest.
To achieve this goal the team works at the interface of Organometallics, coordination chemistry and catalysis, for the design of new active molecular metal catalysts especially Ruthenium and recently Rhodium catalysts, the characterization of catalysis intermediates and the understanding of catalytic mechanisms.
Recently, the research topics concentrated more on catalytic C-H bond activation and functionalization |
The successive Research Topics are presented here |
1. Catalytic C-H bond activation and functionalisation
2. Alkene metathesis and renewables, plant oils transformations
3. Catalysis for selective processes and fine chemistry
4. Carbon-rich organometallics |
| |
| |
|
| 1. C-H bond activation / functionalization |
| |
The activation/functionalization of usually inert C-H bonds to reach new cross-coupled C-C and C-Heteroatom bonds is currently our main objective |
Recent discoveries involve |
|
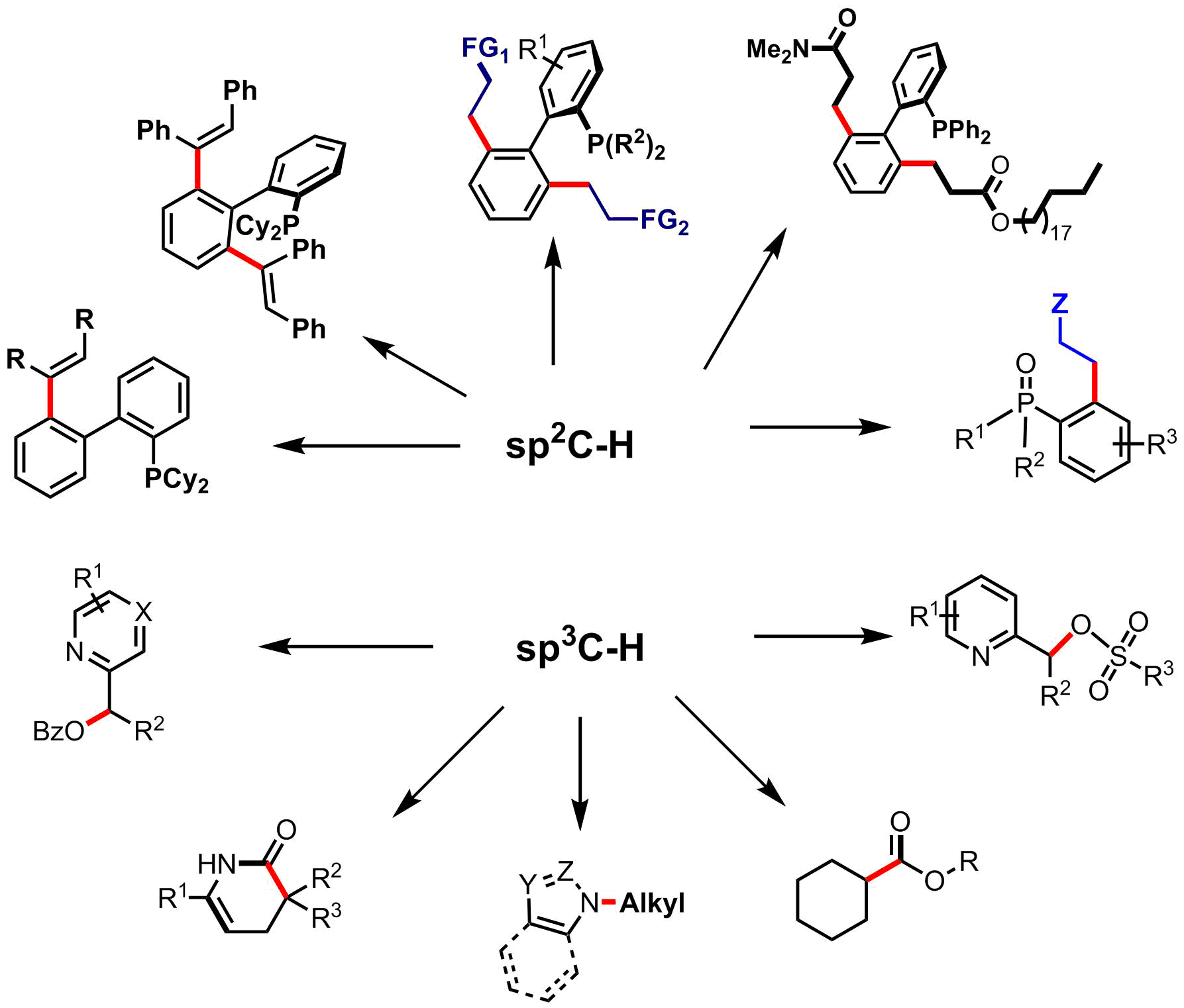
Fast modification of Phosphines and phosphine oxides via C-H bond functionalization, such as selective alkenylation, alkylations, dialkenylations with Ru(II) and Rh(I) catalysts.
Applications were found in cross coupling C-C bond formation and especially in Carboxylation of Aryl-halides with CO2 with modified phosphines which are improving the catalyst efficiency.(With Dr J F Soulé)
Fast modification of 2-alkylpyridine N-oxides via functionalization of alpha sp3C-H bonds of 2-alkyl chain making C-O bonds.
Synthesis via carbonylation of sp3C-H bonds via radical processes with Fe(II) and Cu(II) catalysts (with X F Wu & J F Soulé) for functionalization of alkanes and synthesis of esters, lactams, and cross coupling C-N bonds. |
 |
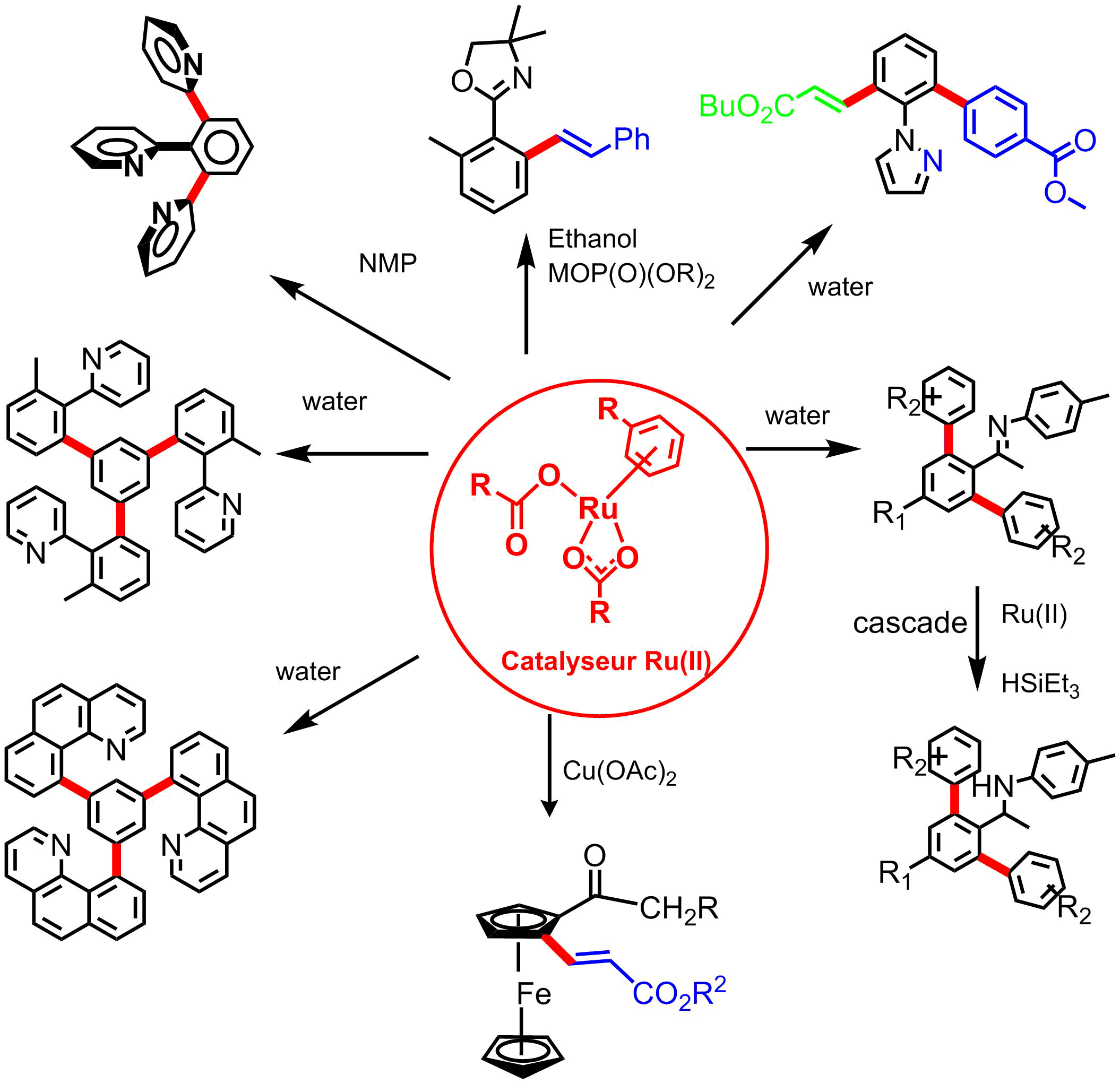
We approached this problem by the use of easy to make and often air and water stable ruthenium(II) catalysts, especially Ru-carbonate and Ru-carboxylate catalysts.
Our Results involve
the direct arylation of functional arenes and heterocycles with (hetero)aryl chlorides
the initial C-H bond activation via C-H bond deprotonation by coordinated ligand,carbonate or external carboxylate and Ru(II) site, via an autocatalytic process.
This is supported by
- DFT calculations by Feliu Maseras (ICIQ Tarragona) and
- kinetics By Anny Jutand (ENS, Paris)
C-H bond functionalization in green solvents and dialkylcarbonates as solvents
C-H bond activation and arylation with aryl chlorides in water
oxidative alkenylation of functional arenes with olefins with addition of Cu(II) catalysts
the synthesis of polyheterocyclic derivatives and polypodal ligands (with Franck Pozgan, Ljubjana Slovenia, Marie Curie post doc) |
|
Reviews on C-H bond activation and functionalization |
-Ruthenium(II) Catalyzed C-H Bond Activation and Functionalization
Percia Beatrice Arockiam, Christian Bruneau, Pierre H. Dixneuf Chem. Rev. 2012, 112 (11), 5879–5918. (Citations > 2100)
- sp2C-H Bond activation in water and catalytic cross-coupling reactions
B. Li , P. H. Dixneuf Chem. Soc. Rev.2013, 42 (13), 5744 - 5767(Citations > 440))
-Late Stage Modifications of P-Containing Ligands using Transition-Metal-Catalysed C–H Bond Functionalisation , Zhuan Zhang, Pierre H Dixneuf and Jean-Francois Soule , Feature Article, Chem. Commun., 2018, 54, 7265 – 7280 DOI: 10.1039/C8CC02821D
-Photoredox Catalysis for Building C−C Bonds from C(sp2)−H Bonds
Chang-Sheng Wang, Pierre H. Dixneuf, and Jean-François Soulé ; Chem. Rev. 2018, 118, 7532-7585. DOI: 10.1021/acs.chemrev.8b00077 (Citations > 320)
-Functionalizations of C(sp2)–H Bonds of Heterocycles and Arenes Assisted with Photoredox-Catalysts for the C–C Bond Formation
P. H. Dixneuf, J.-F. Soulé In "Organometallics for Green Catalysis ", P. H. Dixneuf, J. F. Soulé Eds., Top. OrganoMet. Chem., Springer, 2019,Vol 63, 225 – 265. DOI : 10.1007/3418_2018_22 |
| |
2. Alkene metathesis catalysts and applications from plant oils |
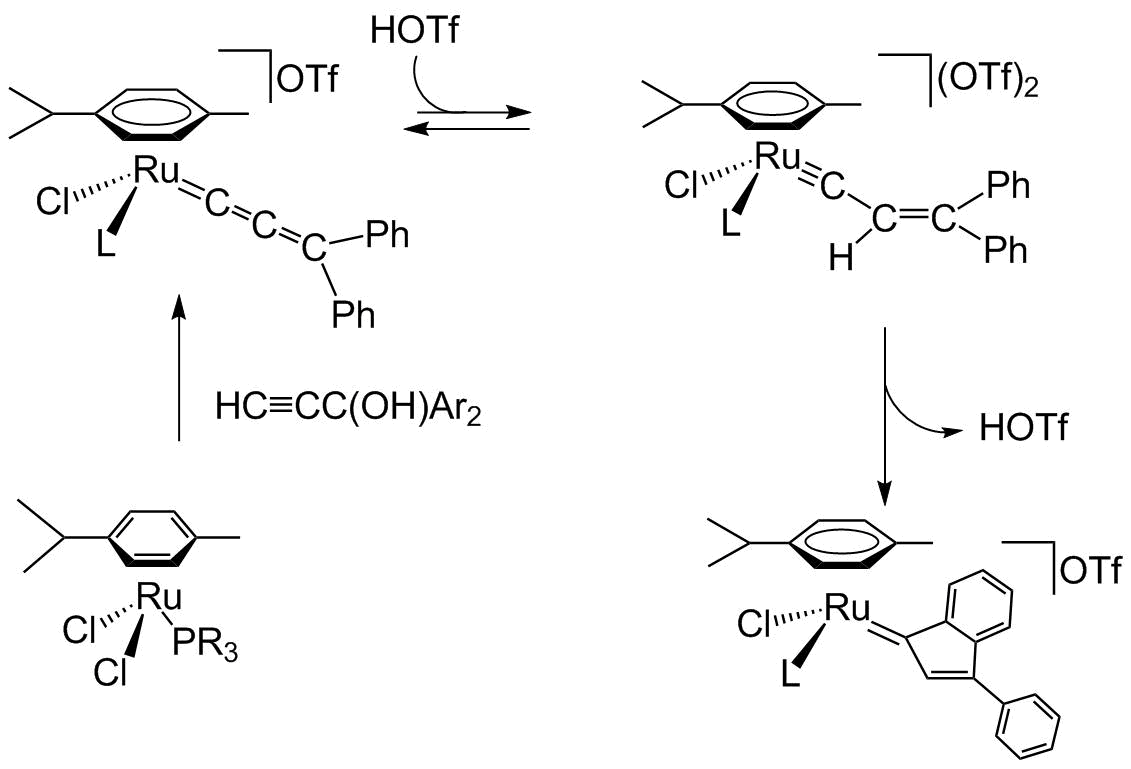
Discovery of Carbon-rich ruthenium catalysts for olefin metathesis, based on ruthenium - allenylidene Ru=C=C=CR2 , the precursors of active ruthenium-indenylidene catalysts and other metathesis catalysts: Ring Closing Metathesis (RCM), catalytic synthesis of heterocycles and fluorine containing derivatives, transformation of natural products and ROMP Polymerisations.
As early publications in the field see |
Highly Active Catalysts in Alkene Metathesis : First Observed Transformation of Allenylidene into Indenylidene via Alkenylcarbyne - Ruthenium Species
R. Castarlenas, C. Vovard, C. Fischmeister, P. H. Dixneuf,
Angew. Chem. Int. Ed. 2003, 42, 4524-4527
Allenylidene to Indenylidene Rearrangement in Arene-Ruthenium Complexes: a Key Step to Highly Active Catalysts for Olefin Metathesis Reactions
R. Castarlenas, C. Vovard, C. Fischmeister, P. H. Dixneuf,
J. Am. Chem. Soc. 2006, 128, 4079-4089 |
 |
See Book Chapter :
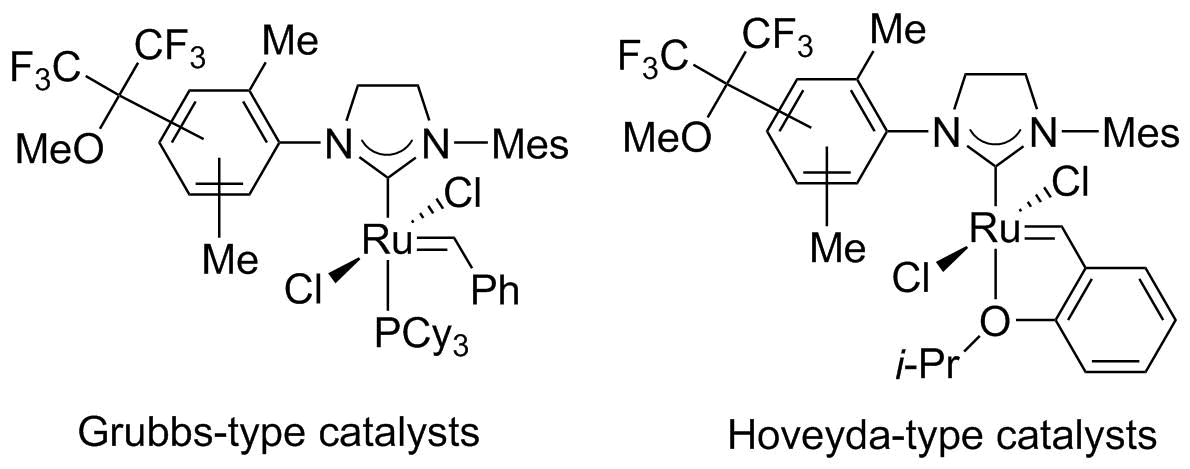
Indenylidene-Ruthenium catalysts for Alkene Metathesis
P. H. Dixneuf, C. Bruneau in "Handbook of Metathesis, Volume 1: Catalyst Development and Mechanism", R. H. Grubbs, A. G. Wenzel Eds., Wiley VCH, Weinheim, 2nd edition, 2015, pp 389-416. |
 |
Alkene metathesis and catalytic transformations of renewables such as terpenes and plant oil derivatives into nitrogen containing and bifunctional products.
Synthesis of linear aminoacids, the precursor of Polyamides, via cross-metathesis of unsaturated fatty esters or nitriles, with acrylonitrile or acrylates and tandem catalytic hydrogenation. |
Alkene metathesis catalysis: a key for transformations of unsaturated plant oils and renewable derivatives.
Pierre H. Dixneuf , Christian Bruneau, Cédric Fischmeister
Oil & Gas Sci. Technol., 2016, in press Dedicated to Yves Chauvin
Transformations of Terpenes via Carbon-Carbon Double Bond Metathesis
Bruneau, christian; Fischmeister, Cédric; Mandelli, Dalmo; Carvalho, Wagner; dos Santos, Eduardo; Dixneuf, pierre; Sarmento Fernandes, Luciana; Catal. Sci. Technol., 2018, 8, 3989-4004, CY-MRV-06-2018-001152.R1 |
| |
| 3. Catalysis for selective processes and fine chemistry: Catalytic transformations of alkynes and development of ruthenium catalysts |
New selective catalytic combinations of simple molecules with atom economy and clean processes.
. Selective catalytic formation of C-C bonds via oxidative couplings of alkynes , diynes and enynes with electron rich Ruthenium(II) catalysts Cp*RuXLn,
Evidence for Bis-Carbene ruthenium intermediates. Catalytic addition of carbenes to alkynes and enynes. (In cooperation with Dr S. Derien) |
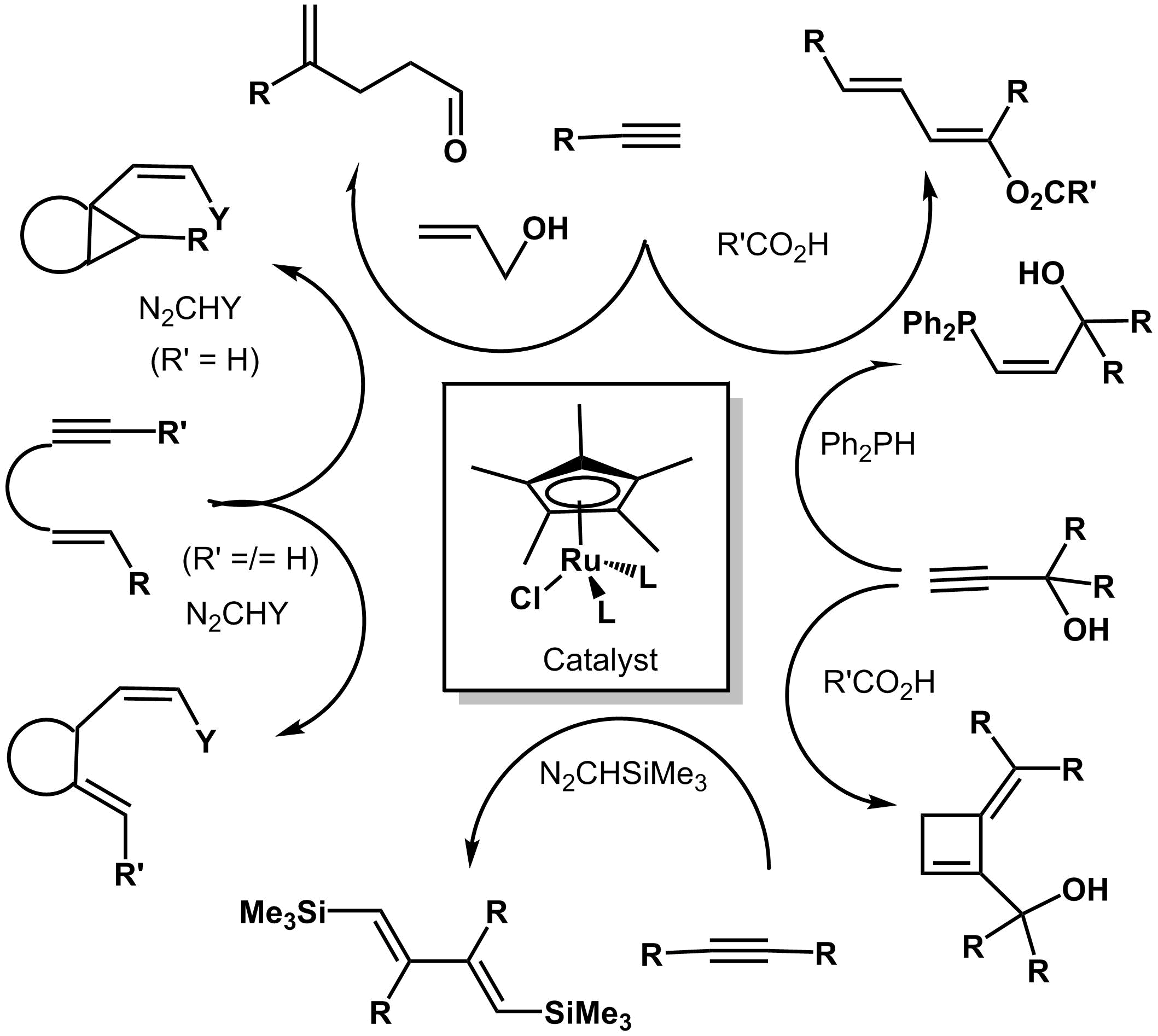 |
See review :
Cp*RuCl(COD) in Catalysis : a Unique Role in the Addition of Diazoalkane Carbene to Alkynes.
C. Vovard- Le Bray, S. Dérien, P. H. Dixneuf,
C. R. Acad Sciences (2010) 13, 292-303 |
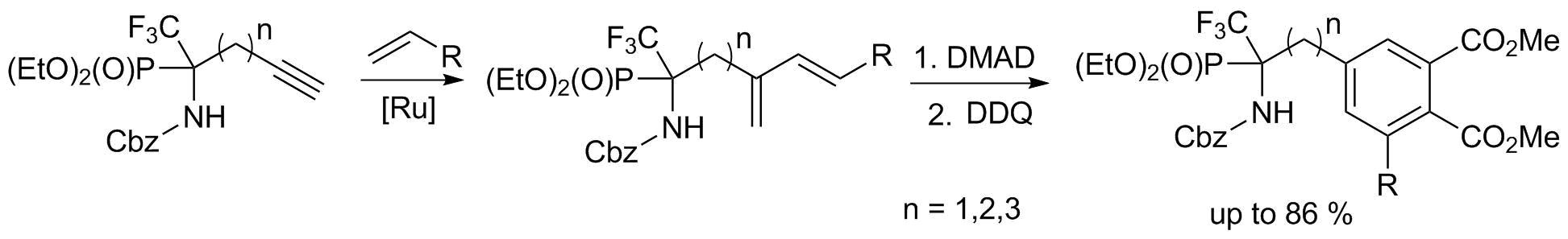
Selective syntheses of fluorinated molecules via activation and cyclisation of fluorinated amino acids or phosphonates.in cooperation with Prof S. Osipov, Ineos Moscow.(PICS, GDRI) |
 |
| |
| Metal-vinylidenes in catalysis |
Anti-Markovnikov additions to the terminal alkynes: catalytic synthesis of enol esters, vinylcarbamates, hydrophosphination of alkynes.
First evidence of vinylidene-metal intermediate in Catalysis
Characterisation of metal-vinylidenes, allenylidenes intermediates. |
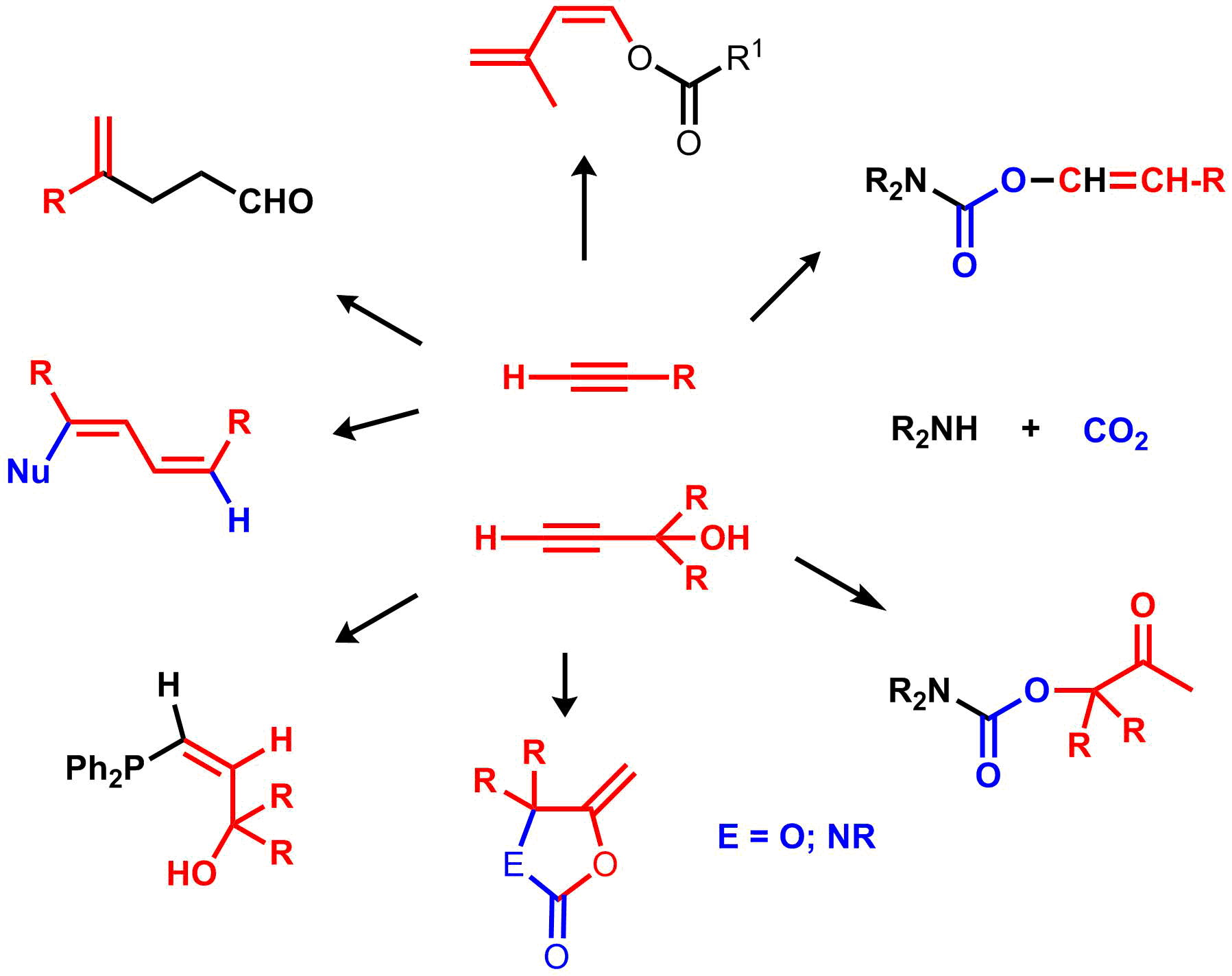 |
See reviews :
Metal vinylidenes and allenylidenes in catalysis. Applications in anti Markovnikov additions to terminal alkynes and alkene metathesis
C. Bruneau, P. H. Dixneuf,
Angew. Chem. Int. Ed., 2006, 45, 2176-2203.
Early steps of homogeneous catalysis in Rennes: carbon dioxide incorporation, alkyne activation and ruthenium catalysis.
Pierre H. Dixneuf, Catal. Lett., 2015, 145, 360–372 |
| |
4. Novel Carbon-rich organometallics and catalyst models |
Novel highly unsaturated organoruthenium systems containing carbon-rich chains have been prepared for the discovery of new physical properties, the building of organometallic monomers, communication through carbon-rich bridges, and as models of ruthenium based catalysts, especially vinylidene- and allenylidene- ruthenium catalysts.
The stoichiometric activation of functional alkynes and polyynes leads to mono- and poly-vinylidenes Ru=C=CHR and allenylidenes Ru=C=C=CR2, complexes higher metallacumulenes Ru=(C=)nCR2, bimetallic systems with carbon-rich bridges, multipodal polyynes and polymetallic metal complexes. |
Early publications and review:
Ruthenium-allenylidene complexes and their specific behaviour.
S. Rigaut, D. Touchard, P. H. Dixneuf,
Coord. Chem. Rev., 2004, 248, 1585-1601.
Ruthenium Containing Cumulenes: Generation of 2,3,4- Pentatrienylidene- and 3-Oxo-1,4-Pentadienyl- complexes.
A. ROMERO, A. VEGAS, and P. H. DIXNEUF, Angew. Chem. Int. Ed. Engl., 1990, 29, 215-216.
This topic, initiated with prof Daniel Touchard, is now developed by Stéphane Rigaut in Rennes. |
| |
| Funding |
CNRS, Ministère de l'Enseignement Supérieur et de la Recherche,
Université de Rennes, Région Bretagne, Institut universitaire de France (IUF),
European Union (Marie Curie, Various Networks HCM, IDECAT Network), ANR (National Agency for Research)
Industry support for 25 years : SNPE, Oril Industrie, IFP, Firmenich, Arkema |
| |















